Patients who need more than what a statin alone can provide, or those unable to take recommended statin therapy may be right for NEXLIZET or NEXLETOL1,2
NEXLIZET and NEXLETOL are the only nonstatins FDA approved to lower LDL-C and reduce the risk of MI and coronary revascularization in primary prevention and secondary prevention patients.1,2
Learn more by choosing one of the patients below:
Primary prevention with diabetes
Secondary prevention, prior MI
Primary prevention, cannot take a statin, elevated risk score
Approved to reduce the risk of MI and coronary revascularization,
NEXLIZET may be right for Naomi, a primary prevention
patient who has diabetes and is taking a statin1,2
Naomi is at risk for her first cardiac event due to2:
- Current diagnosis of type 2 diabetes
- Hypertension
She is currently taking atorvastatin 20 mg.
Age: 65
BMI: 33 kg/m2

Hypothetical patient.
Despite being a busy grandmother of 6,
Naomi still finds time to direct the choir
at her local church.
Despite being a busy grandmother of 6,
Naomi still finds time to direct the choir
at her local church.
In the CLEAR Outcomes Trial, which
included both primary prevention
and secondary prevention patients,
bempedoic acid, a component
of NEXLIZET, demonstrated
a 27% relative risk reduction for
nonfatal MI vs placebo.2
HR, 0.73 (95% CI: 0.62-0.87)
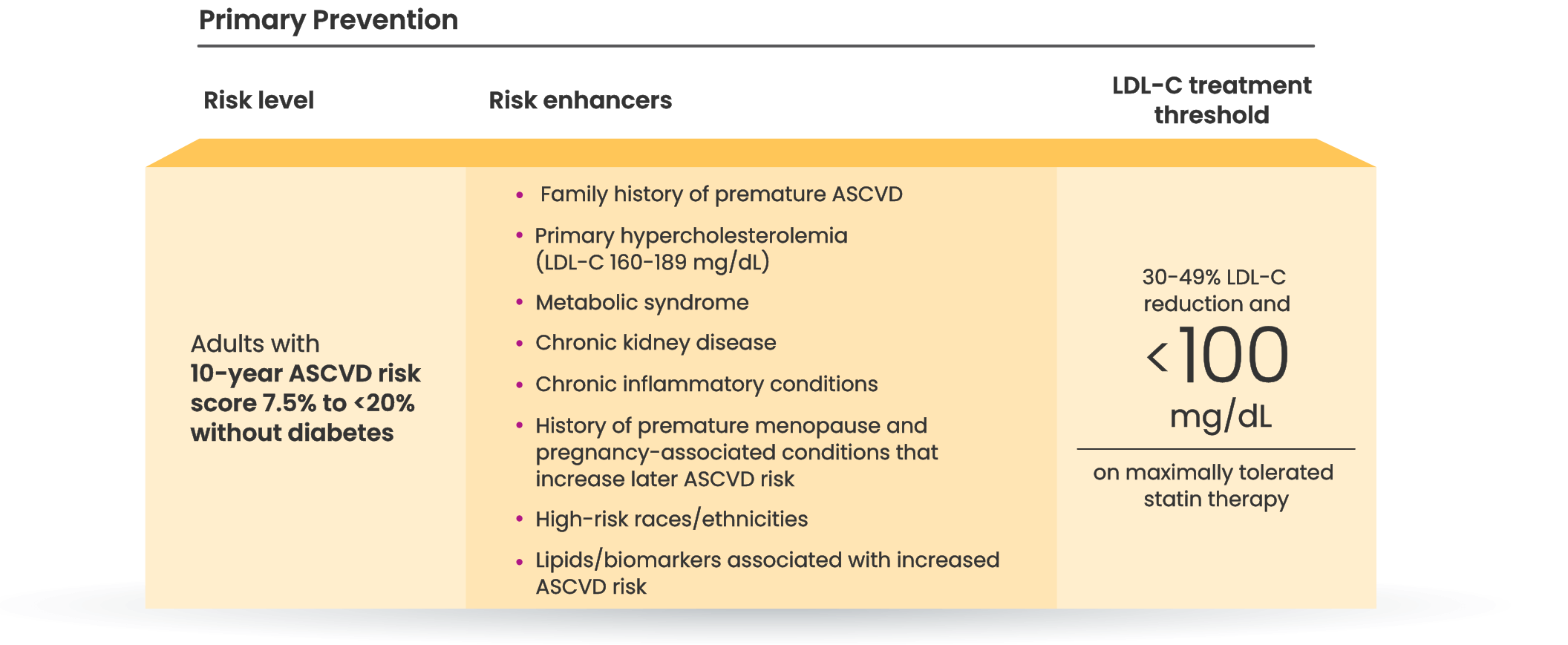
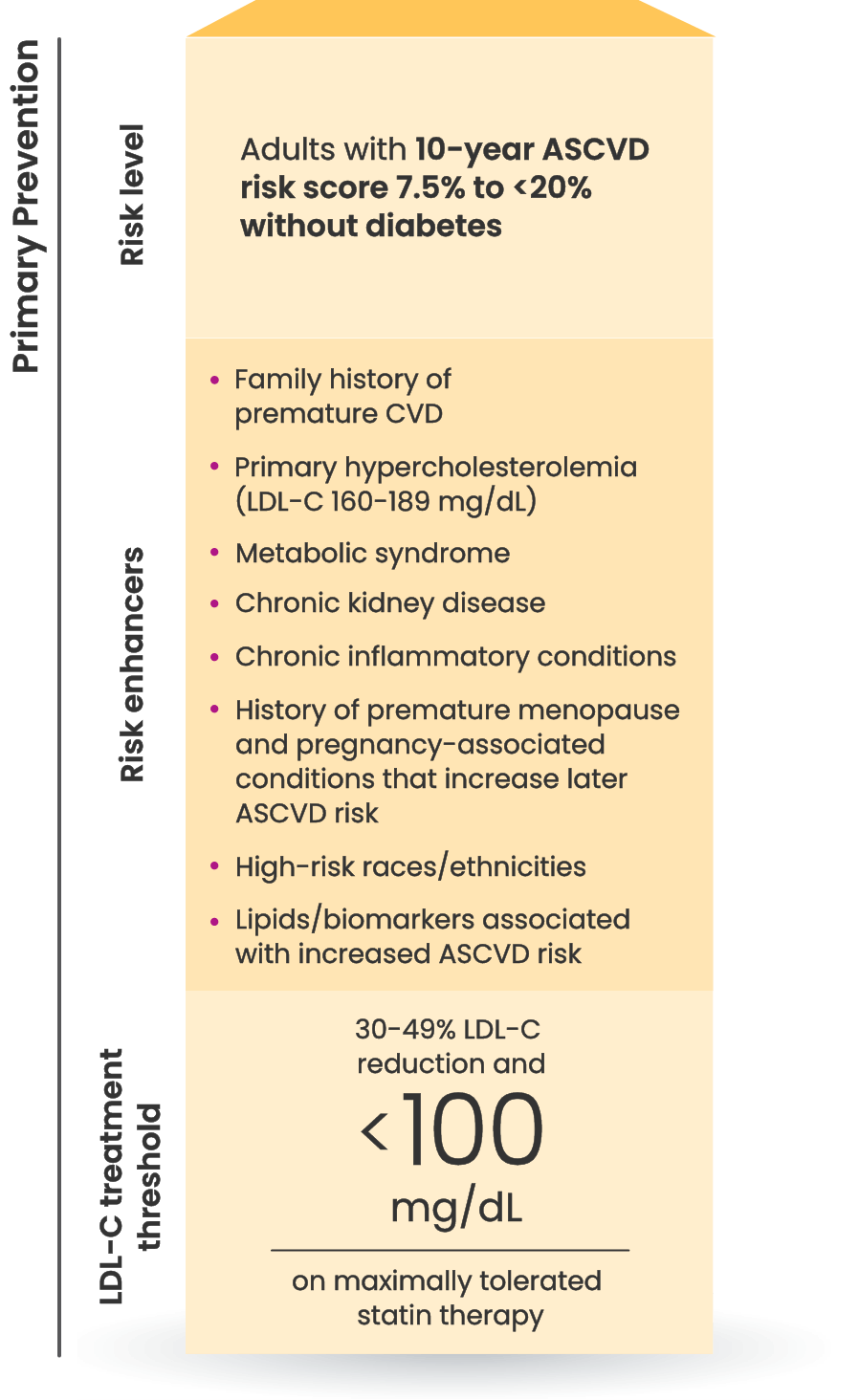
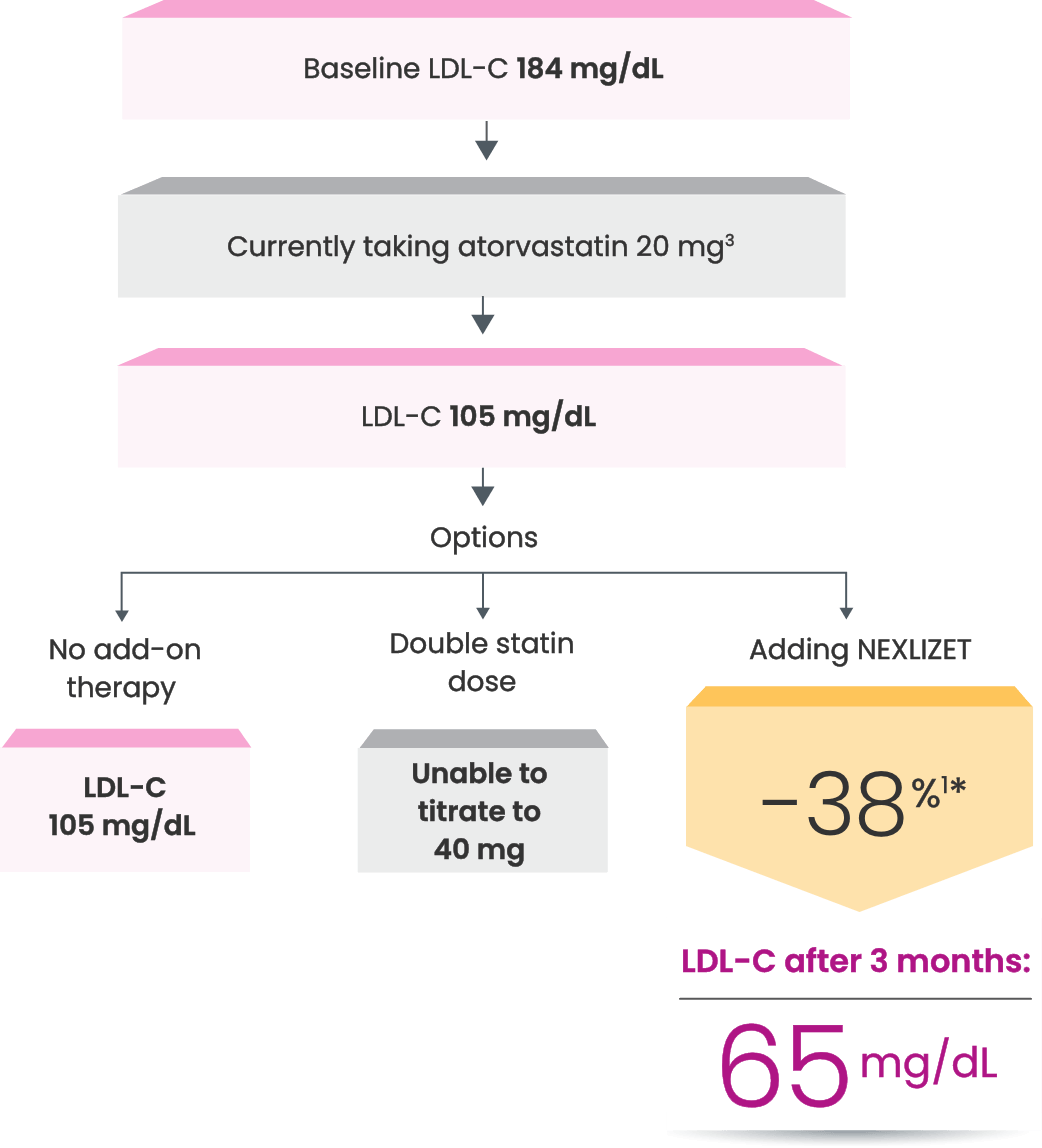
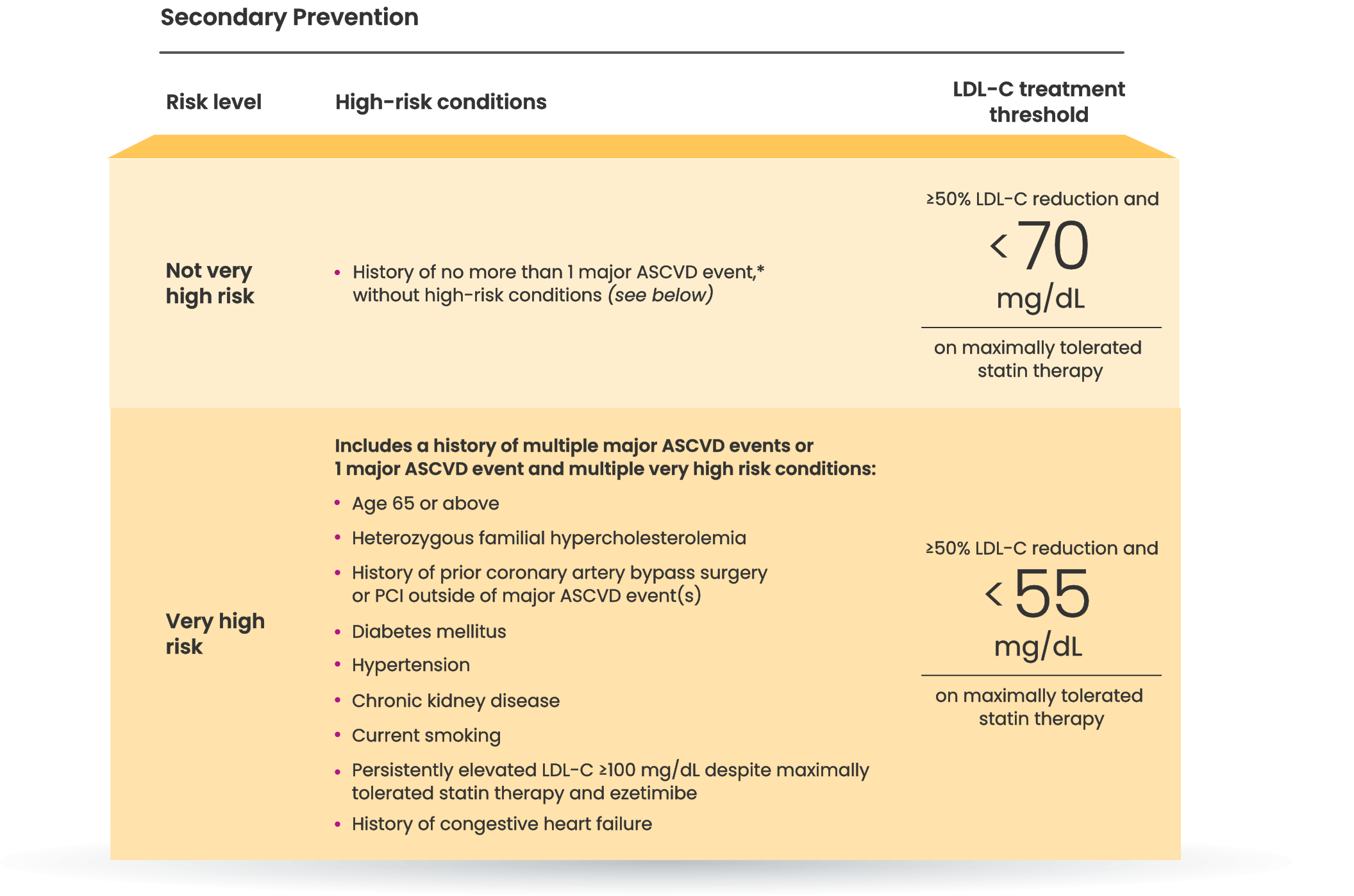
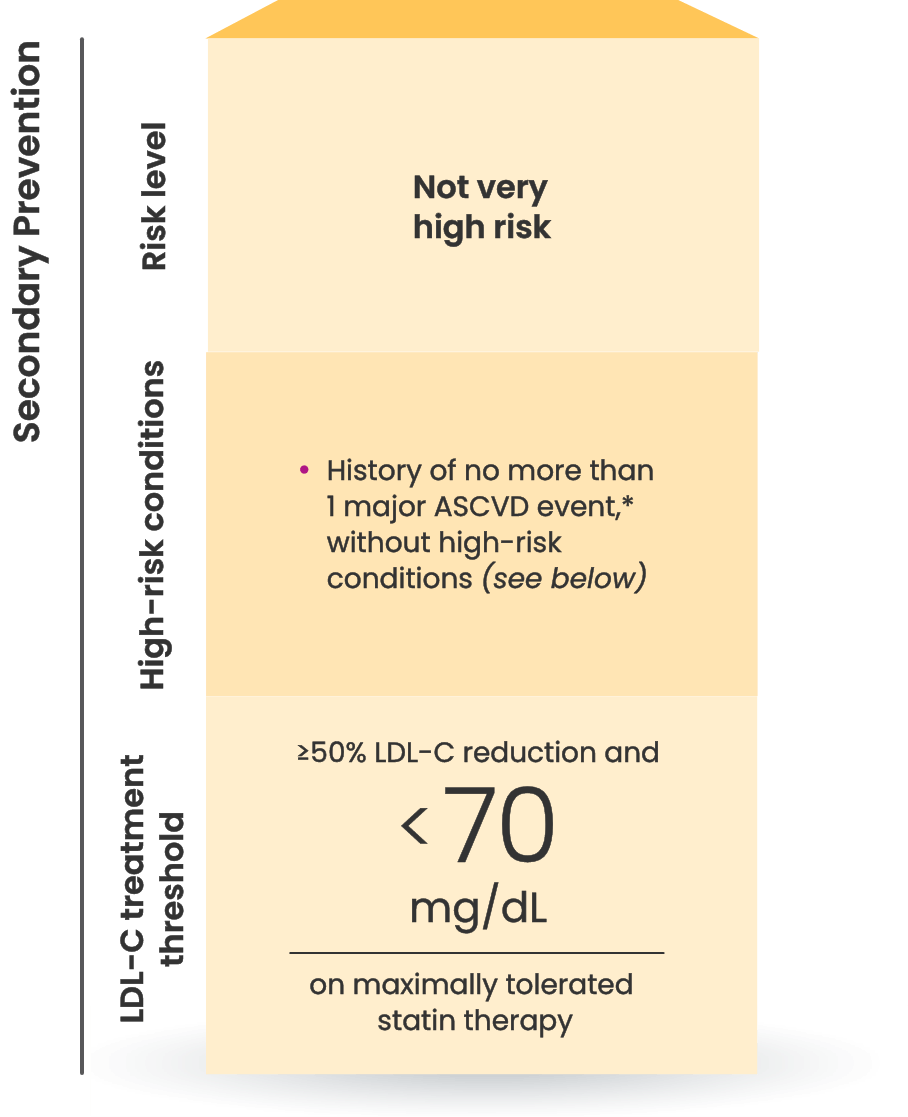
Many primary prevention patients need additional treatment
to lower LDL-C and reduce CV risk4,5
LDL-C treatment threshold recommendations based on the 2022 ACC Expert Consensus Decision Pathway6
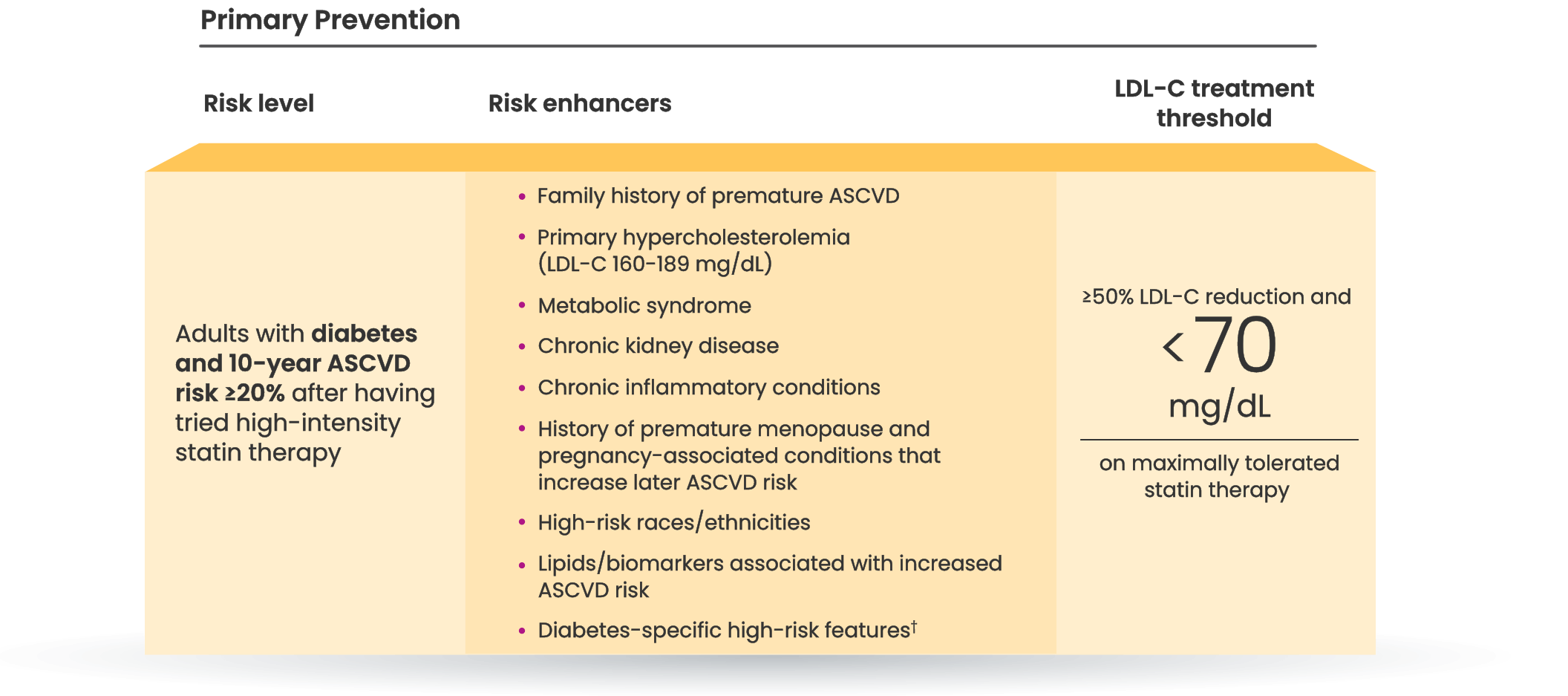
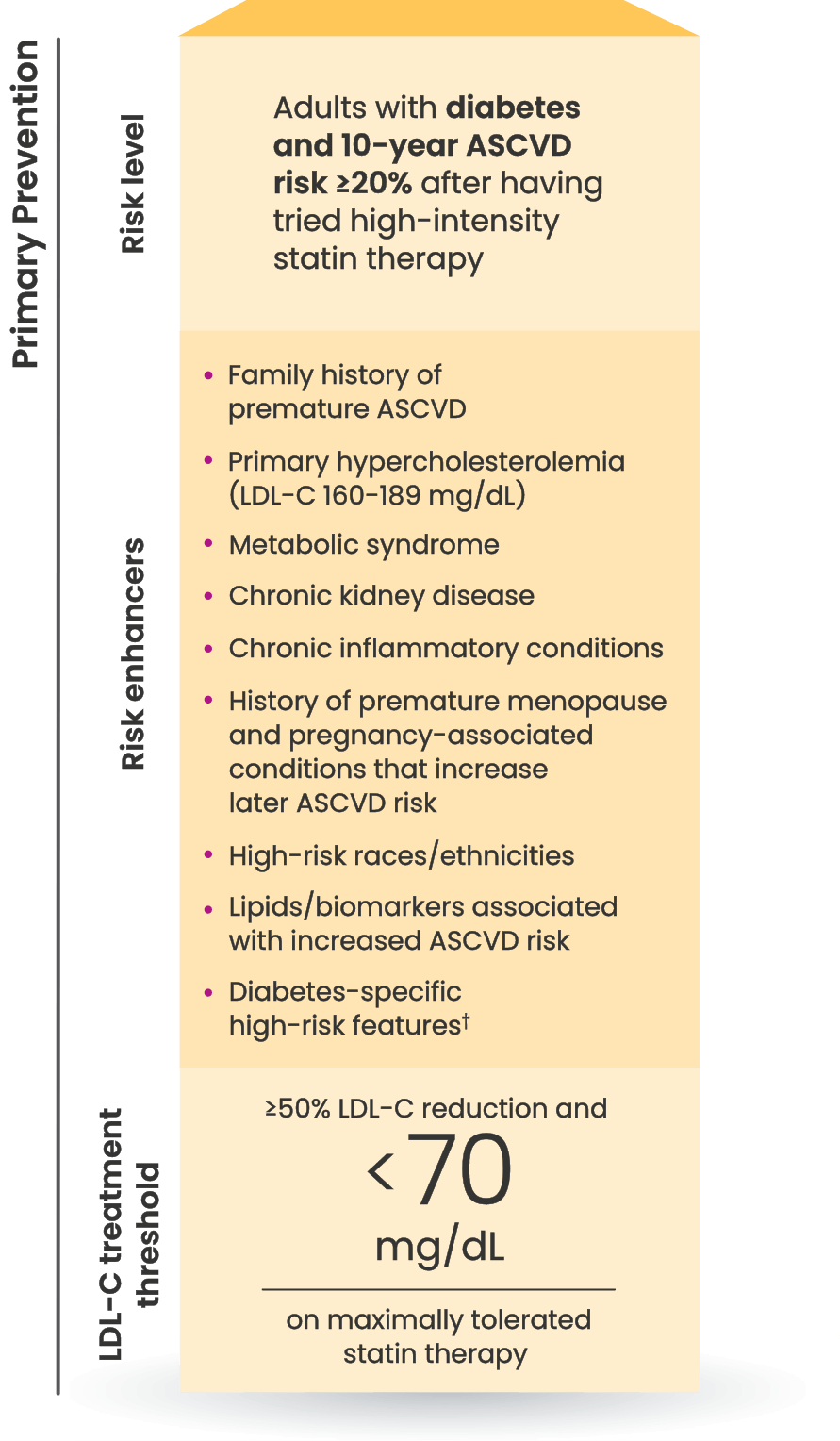
†Long duration (≥10 years for type 2 diabetes or ≥20 years for type 1 diabetes), albuminuria ≥30 mcg of albumin/mg creatinine, eGFR <60 mL/min/1.73 m2, retinopathy, neuropathy, ABI <0.9.6
46.8%
of people at intermediate or
high risk for a first ASCVD event
are already on a statin but not at LDL-C goal.7
Who else is right for NEXLIZET or NEXLETOL?
Nick, a secondary prevention patient with a prior MI1,2
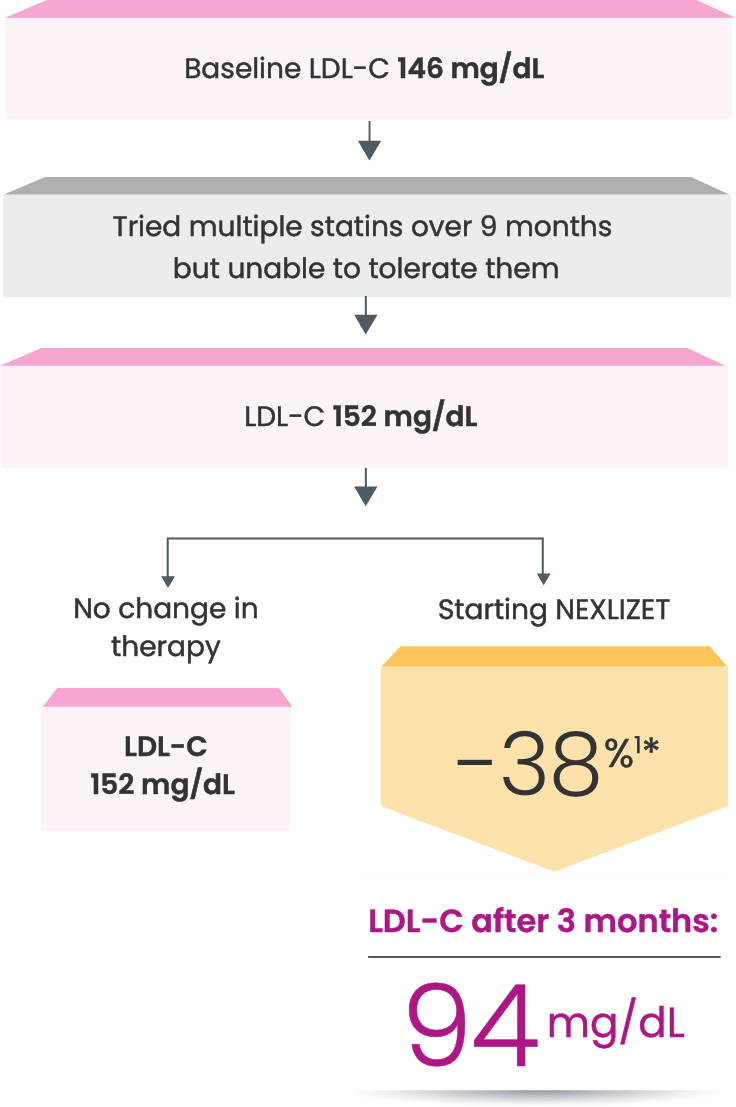
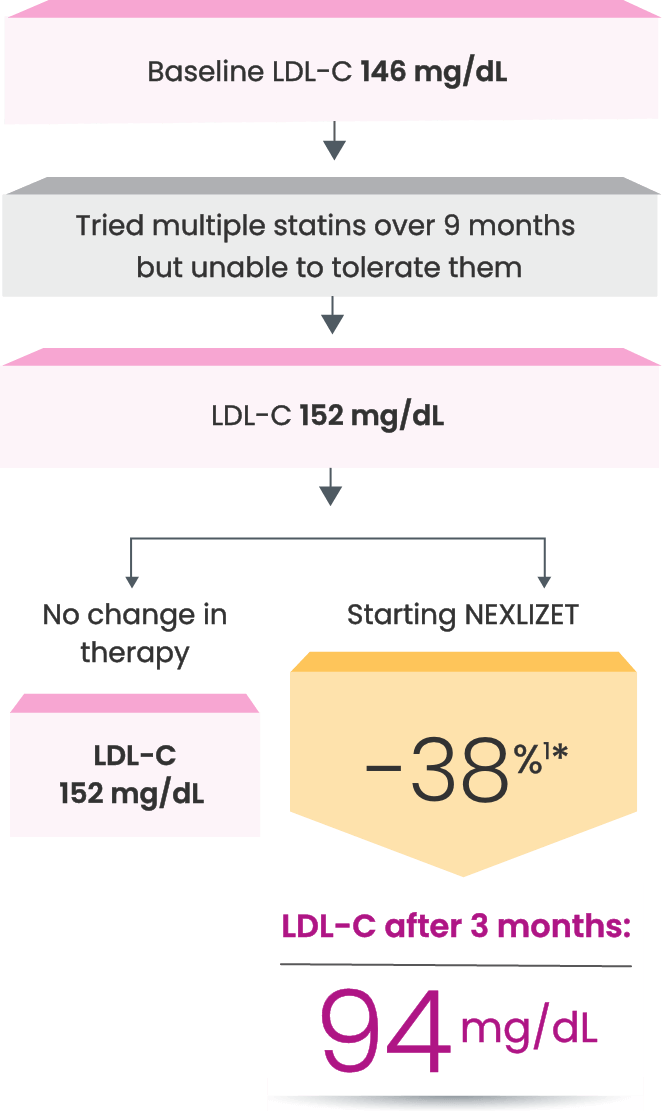
*Fictitious patient. Individual results may vary. Results shown are based on a mean 38% placebo-corrected LDL-C reduction from baseline (-36% NEXLIZET vs +2% placebo) at 12 weeks.1
Approved to reduce the risk of MI and coronary revascularization,
NEXLIZET may be right for Nick, a secondary prevention patient
who needs more than a statin alone1,2
Nick is at risk for another cardiac event due to2:
- MI that occurred 1 year ago
- Hypertension
- Elective PCI 3 years ago
- A1C 6.3%
He is currently taking rosuvastatin 20 mg.
Age: 62
BMI: 35 kg/m2

Hypothetical patient.
Since he retired, Nick spends a lot of time
volunteering at the local library and is excited
to become a grandfather for the first time.
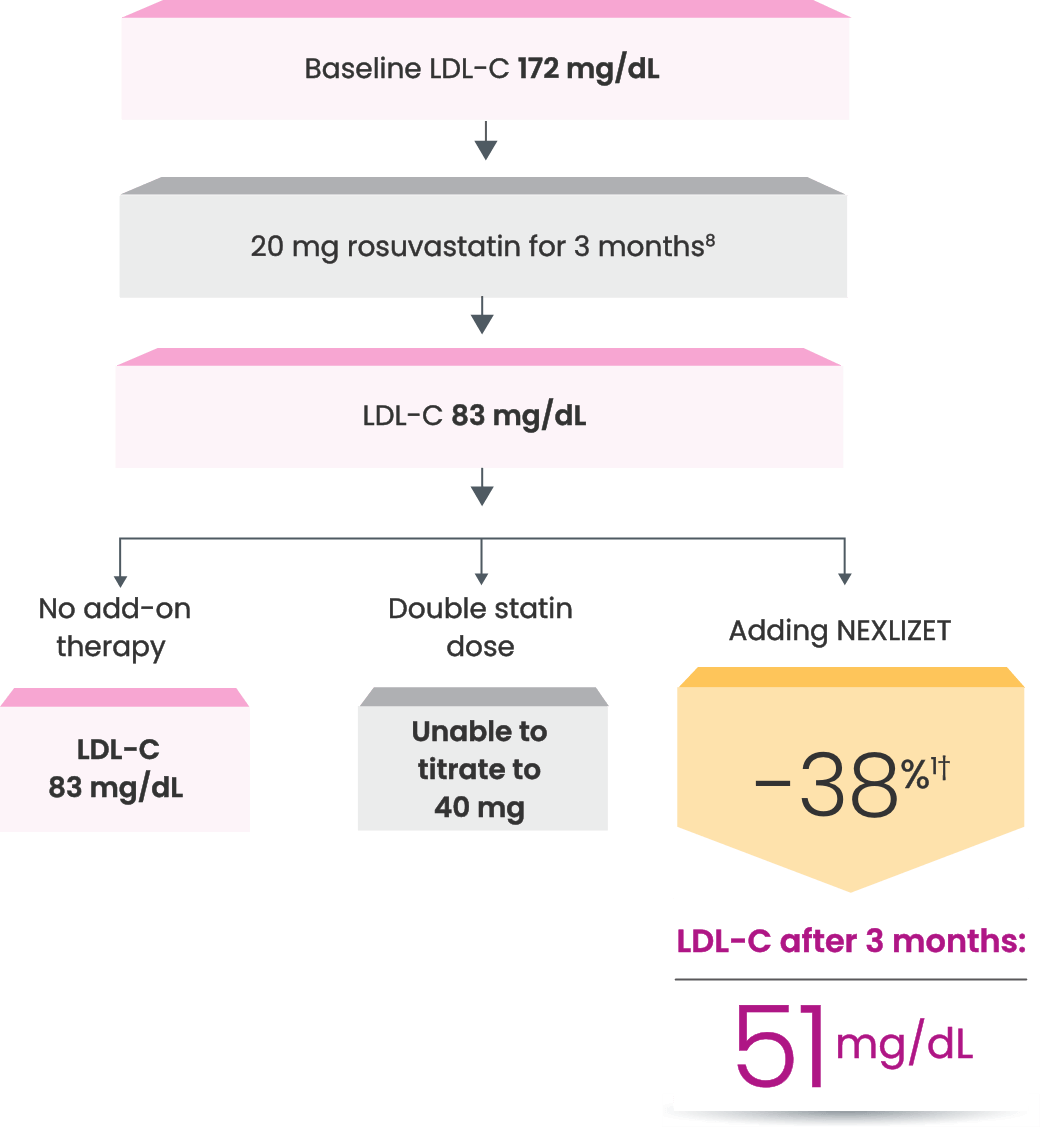
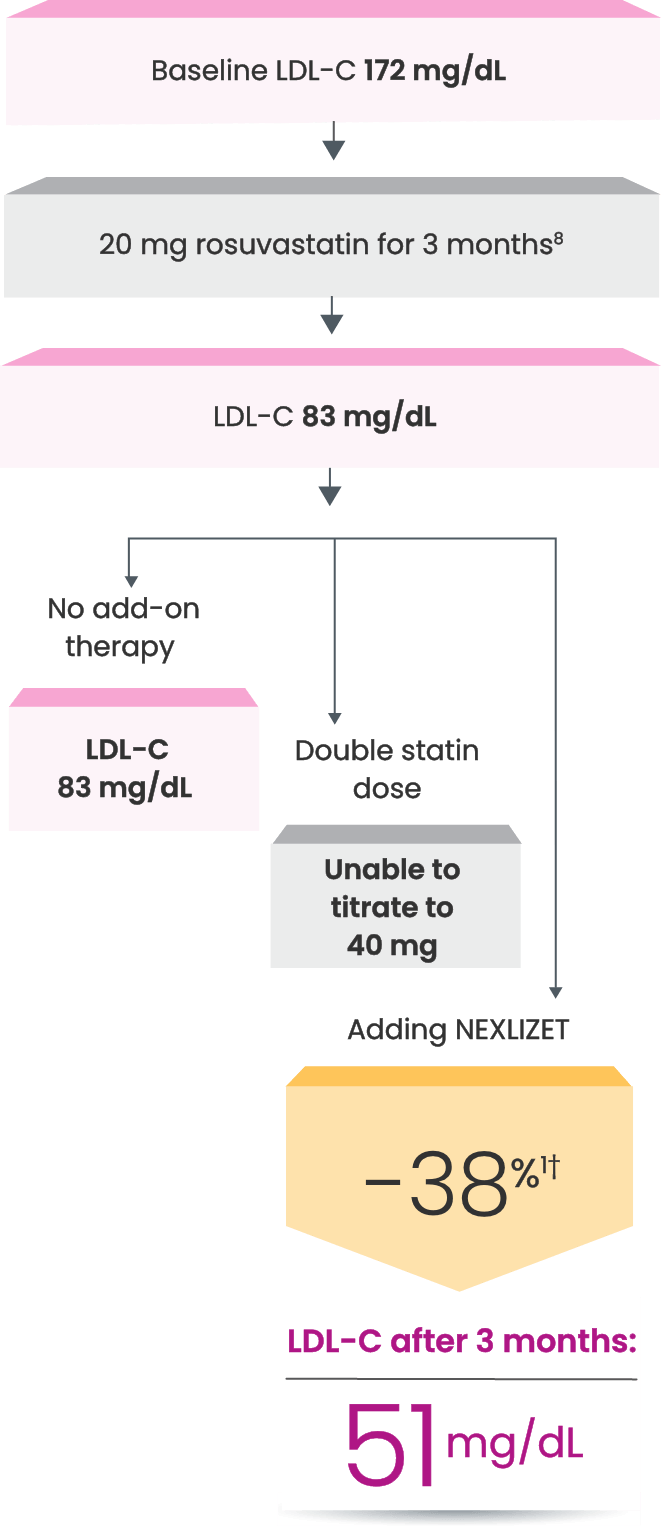
Since he retired, Nick spends a lot of time
volunteering at the local library and is excited
to become a grandfather for the first time.
In the CLEAR Outcomes Trial, which
included both primary prevention
and secondary prevention patients,
bempedoic acid, a component
of NEXLIZET, demonstrated
a 27% relative risk reduction for
nonfatal MI vs placebo.2
HR, 0.73 (95% CI: 0.62-0.87)
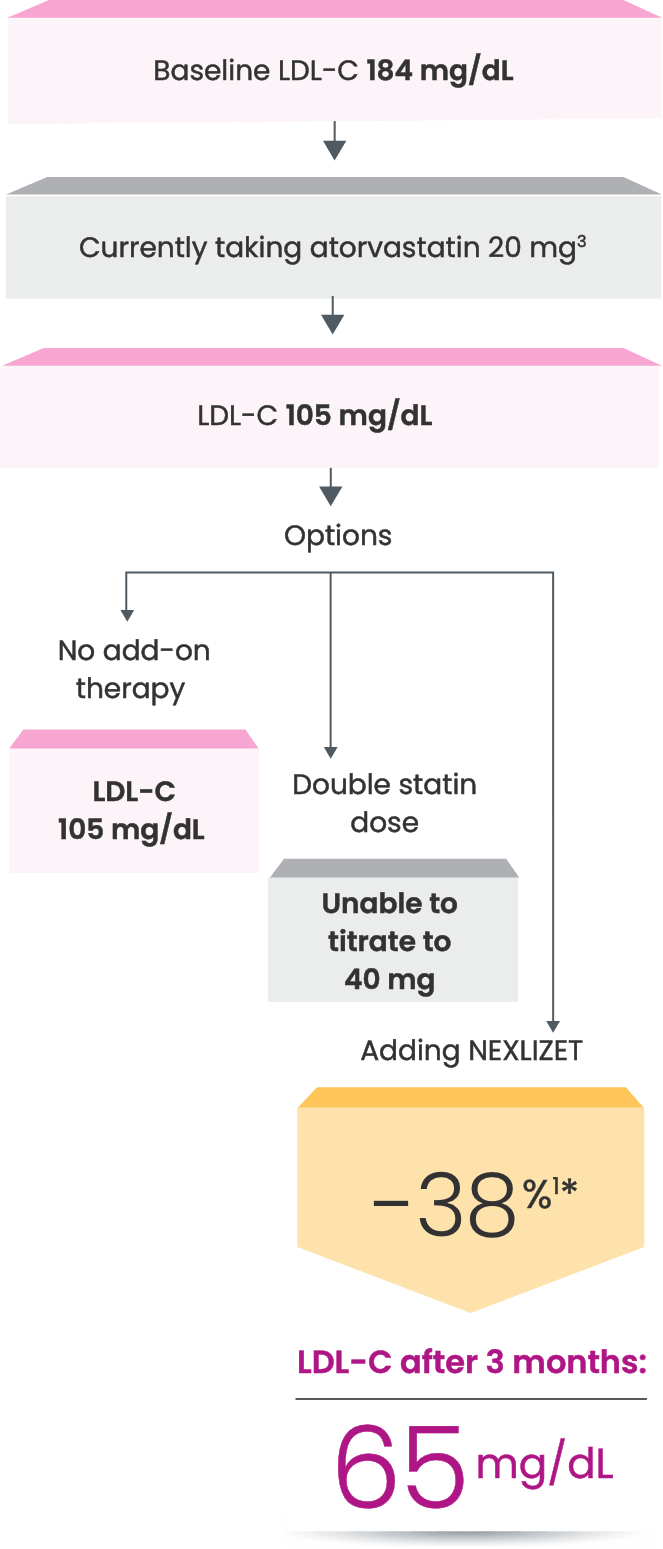
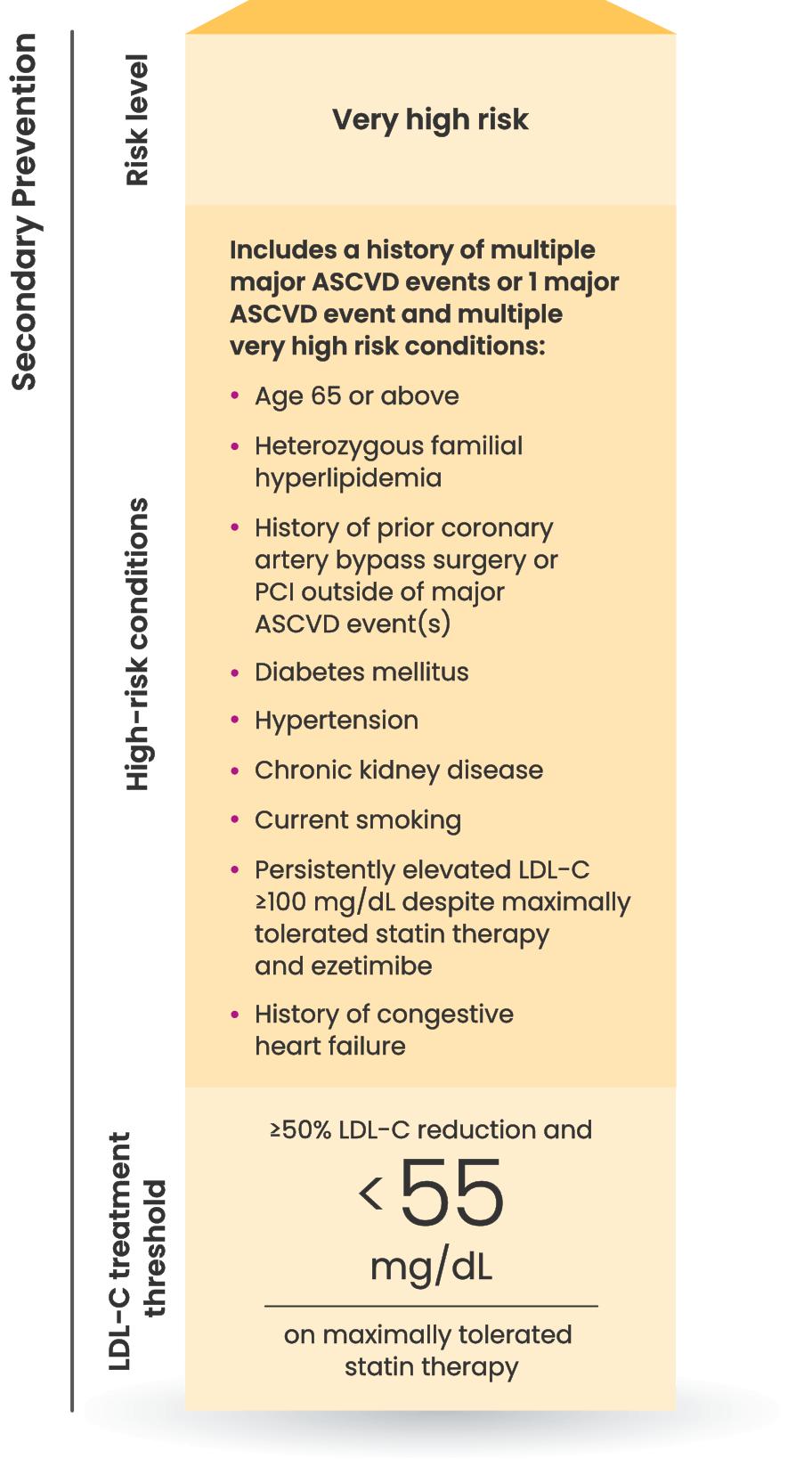
Many CVD patients need additional treatment
to lower LDL-C and reduce CV risk7
LDL-C treatment threshold recommendations based on the 2022 ACC Expert Consensus Decision Pathway6
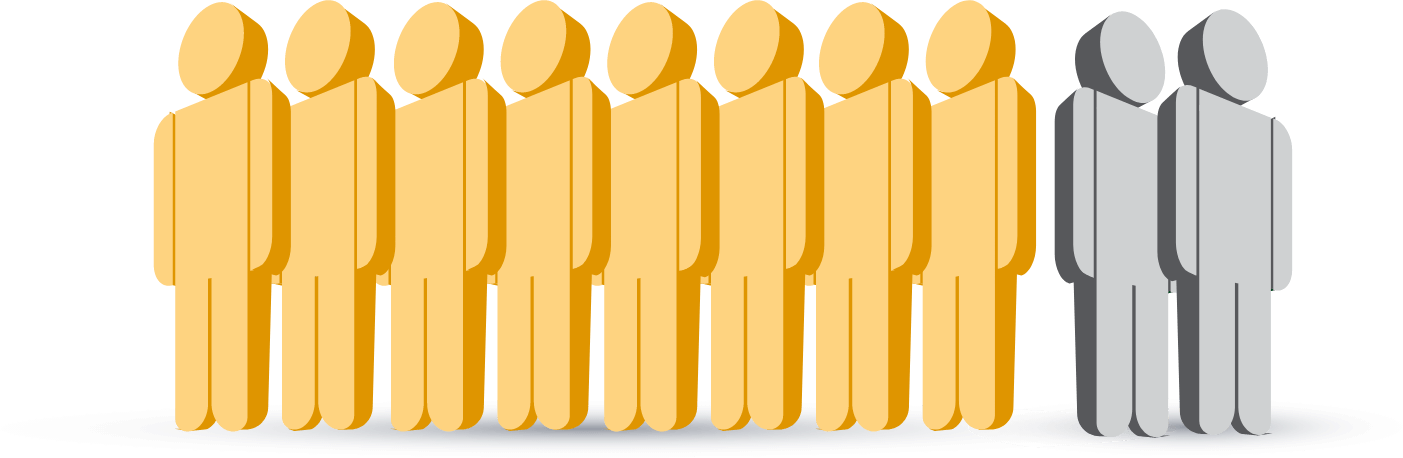
8 out of 10 patients on a
statin with ASCVD
are at risk for another CV event
because they are not at LDL-C goal.7
Who else is right for NEXLIZET or NEXLETOL?
Nora, a primary prevention patient unable to take a statin1,2
*Fictitious patient. Individual results may vary. Results shown are based on a mean 38% placebo-corrected LDL-C reduction from baseline (-36% NEXLIZET vs +2% placebo) at 12 weeks.1
†Major ASCVD events include fatal and nonfatal MI, recent ACS (within the past 12 months), history of MI (other than recent ACS event), history of ischemic stroke, symptomatic PAD (history of claudication with ABI <0.85 or previous revascularization or amputation).6

Interested in samples?
Get patients started with a 14-day sample of NEXLIZET or NEXLETOL.
Request samplesINDICATION AND IMPORTANT SAFETY INFORMATION
Indication
NEXLIZET and NEXLETOL are indicated:
- The bempedoic acid component of NEXLIZET and NEXLETOL is indicated to reduce the risk of myocardial infarction and coronary revascularization in adults who are unable to take recommended statin therapy (including those not taking a statin) with:
- established cardiovascular disease (CVD), or
- at high risk for a CVD event but without established CVD.
- As an adjunct to diet:
- NEXLIZET, alone or in combination with other LDL-C lowering therapies, to reduce LDL-C in adults with primary hyperlipidemia, including HeFH.
- NEXLETOL, in combination with other LDL-C lowering therapies, or alone when concomitant LDL-C lowering therapy is not possible, to reduce LDL-C in adults with primary hyperlipidemia, including HeFH.
IMPORTANT SAFETY INFORMATION
NEXLIZET and NEXLETOL are contraindicated in patients with a prior hypersensitivity to bempedoic acid or ezetimibe or any of the excipients. Serious hypersensitivity reactions including anaphylaxis, angioedema, rash, and urticaria have been reported.
Hyperuricemia : Bempedoic acid, a component of NEXLIZET and NEXLETOL, may increase blood uric acid levels, which may lead to gout. Hyperuricemia may occur early in treatment and persist throughout treatment, returning to baseline following discontinuation of treatment. Assess uric acid levels periodically as clinically indicated. Monitor for signs and symptoms of hyperuricemia, and initiate treatment with urate-lowering drugs as appropriate.
Tendon Rupture : Bempedoic acid, a component of NEXLIZET and NEXLETOL, is associated with an increased risk of tendon rupture or injury. Tendon rupture may occur more frequently in patients over 60 years of age, in those taking corticosteroid or fluoroquinolone drugs, in patients with renal failure, and in patients with previous tendon disorders. Discontinue NEXLIZET or NEXLETOL at the first sign of tendon rupture. Consider alternative therapy in patients who have a history of tendon disorders or tendon rupture.
The most common adverse reactions in the primary hyperlipidemia trials of bempedoic acid, a component of NEXLIZET and NEXLETOL, in ≥2% of patients and greater than placebo were upper respiratory tract infection, muscle spasms, hyperuricemia, back pain, abdominal pain or discomfort, bronchitis, pain in extremity, anemia, and elevated liver enzymes.
Adverse reactions reported in ≥2% of patients treated with ezetimibe (a component of NEXLIZET) and at an incidence greater than placebo in clinical trials were upper respiratory tract infection, diarrhea, arthralgia, sinusitis, pain in extremity, fatigue, and influenza.
In the primary hyperlipidemia trials of NEXLIZET, the most commonly reported adverse reactions (incidence ≥3% and greater than placebo) observed with NEXLIZET, but not observed in clinical trials of bempedoic acid or ezetimibe, were urinary tract infection, nasopharyngitis, and constipation.
The most common adverse reactions in the cardiovascular outcomes trial for bempedoic acid, a component of NEXLIZET and NEXLETOL, at an incidence of ≥2% and 0.5% greater than placebo were hyperuricemia, renal impairment, anemia, elevated liver enzymes, muscle spasms, gout, and cholelithiasis.
Concomitant use of NEXLIZET or NEXLETOL with greater than 20 mg of simvastatin or 40 mg of pravastatin should be avoided due to the potential for increased risk of simvastatin- or pravastatin-related myopathy.
Discontinue NEXLIZET or NEXLETOL when pregnancy is recognized unless the benefits of therapy outweigh the potential risks to the fetus. Because of the potential for serious adverse reactions in a breast-fed infant, breastfeeding is not recommended during treatment with NEXLIZET or NEXLETOL.
Report pregnancies to Esperion Therapeutics, Inc. Adverse Event reporting line at at 1-833-377-7633.
Please see full Prescribing Information for NEXLIZET and NEXLETOL.
INDICATION AND IMPORTANT SAFETY INFORMATION
Indication
NEXLIZET and NEXLETOL are indicated:
- The bempedoic acid component of NEXLIZET and NEXLETOL is indicated to reduce the risk of myocardial infarction and coronary revascularization in adults who are unable to take recommended statin therapy (including those not taking a statin) with:
- established cardiovascular disease (CVD), or
- at high risk for a CVD event but without established CVD.
- As an adjunct to diet:
- NEXLIZET, alone or in combination with other LDL-C lowering therapies, to reduce LDL-C in adults with primary hyperlipidemia, including HeFH.
- NEXLETOL, in combination with other LDL-C lowering therapies, or alone when concomitant LDL-C lowering therapy is not possible, to reduce LDL-C in adults with primary hyperlipidemia, including HeFH.
IMPORTANT SAFETY INFORMATION
NEXLIZET and NEXLETOL are contraindicated in patients with a prior hypersensitivity to bempedoic acid or ezetimibe or any of the excipients. Serious hypersensitivity reactions including anaphylaxis, angioedema, rash, and urticaria have been reported.
Hyperuricemia : Bempedoic acid, a component of NEXLIZET and NEXLETOL, may increase blood uric acid levels, which may lead to gout. Hyperuricemia may occur early in treatment and persist throughout treatment, returning to baseline following discontinuation of treatment. Assess uric acid levels periodically as clinically indicated. Monitor for signs and symptoms of hyperuricemia, and initiate treatment with urate-lowering drugs as appropriate.
Tendon Rupture : Bempedoic acid, a component of NEXLIZET and NEXLETOL, is associated with an increased risk of tendon rupture or injury. Tendon rupture may occur more frequently in patients over 60 years of age, in those taking corticosteroid or fluoroquinolone drugs, in patients with renal failure, and in patients with previous tendon disorders. Discontinue NEXLIZET or NEXLETOL at the first sign of tendon rupture. Consider alternative therapy in patients who have a history of tendon disorders or tendon rupture.
The most common adverse reactions in the primary hyperlipidemia trials of bempedoic acid, a component of NEXLIZET and NEXLETOL, in ≥2% of patients and greater than placebo were upper respiratory tract infection, muscle spasms, hyperuricemia, back pain, abdominal pain or discomfort, bronchitis, pain in extremity, anemia, and elevated liver enzymes.
Adverse reactions reported in ≥2% of patients treated with ezetimibe (a component of NEXLIZET) and at an incidence greater than placebo in clinical trials were upper respiratory tract infection, diarrhea, arthralgia, sinusitis, pain in extremity, fatigue, and influenza.
In the primary hyperlipidemia trials of NEXLIZET, the most commonly reported adverse reactions (incidence ≥3% and greater than placebo) observed with NEXLIZET, but not observed in clinical trials of bempedoic acid or ezetimibe, were urinary tract infection, nasopharyngitis, and constipation.
The most common adverse reactions in the cardiovascular outcomes trial for bempedoic acid, a component of NEXLIZET and NEXLETOL, at an incidence of ≥2% and 0.5% greater than placebo were hyperuricemia, renal impairment, anemia, elevated liver enzymes, muscle spasms, gout, and cholelithiasis.
Concomitant use of NEXLIZET or NEXLETOL with greater than 20 mg of simvastatin or 40 mg of pravastatin should be avoided due to the potential for increased risk of simvastatin- or pravastatin-related myopathy.
Discontinue NEXLIZET or NEXLETOL when pregnancy is recognized unless the benefits of therapy outweigh the potential risks to the fetus. Because of the potential for serious adverse reactions in a breast-fed infant, breastfeeding is not recommended during treatment with NEXLIZET or NEXLETOL.
Report pregnancies to Esperion Therapeutics, Inc. Adverse Event reporting line at at 1-833-377-7633.
Please see full Prescribing Information for NEXLIZET and NEXLETOL.
ABI=ankle-brachial index; ACC=American College of Cardiology; ACS=acute coronary syndrome; ASCVD=atherosclerotic cardiovascular disease; BMI=body mass index; CI=confidence interval; CV=cardiovascular; HeFH=heterozygous familial hypercholesterolemia; HR=hazard ratio; LDL-C=low-density lipoprotein cholesterol; MI=myocardial infarction; PAD=peripheral artery disease; PCI=percutaneous coronary intervention.
REFERENCES
1. NEXLIZET. Prescribing information. Esperion Therapeutics, Inc.; 2024. 2. NEXLETOL. Prescribing information. Esperion Therapeutics, Inc.; 2024. 3. LIPITOR® (atorvastatin) [prescribing information]. Vitaris Inc.; 2022. 4. Handelsman Y, Jellinger PS, Guerin CK, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the management of dyslipidemia and prevention of cardiovascular disease algorithm – 2020 executive summary. Endocr Pract. 2020;26(10):1196-1224. 5. Pinkosky SL, Newton RS, Day EA, et al. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat Commun. 2016;7(13457):1-13. 6. Lloyd-Jones DM, Morris PB, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk. J Am Coll Cardiol. 2022;80(14):1366-1418. 7. Wong ND, Young D, Zhao Y, et al. Prevalence of the American College of Cardiology/American Heart Association statin eligibility groups, statin use, and low-density lipoprotein cholesterol control in US adults using the National Health and Nutrition Examination Survey 2011–2012. J Clin Lipidol. 2016;10:1109-1118. 8. Nissen SE, Lincoff DB, Ray KK, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. 2023;388:1353-1364. 9. CRESTOR® (rosuvastatin) [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2023. 10. Pinkosky SL, Filippov S, Srivastava RA, et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J Lipid Res. 2013;54(1):134-151. 11. Cheeley MK, Saseen JJ, Agarwala A, et al. NLA scientific statement on statin intolerance: a new definition and key considerations for ASCVD risk reduction in the statin intolerant patient. J Clin Lipidol. 2022;16:361-375.